With top-notch R&D capabilities, OncoTherapy is working on various solutions in the unstinting fight against cancer worldwide.

In the Japanese medical sector, one of its biggest criticisms is its slow regulatory process, which, on average, is three-to-five years slower compared to Europe or America. However, we have seen some changes since 2012 with the introduction of the Sakigake system and the PMD Act. From your point of view, how would you rate Japan's regulatory framework, and what are some of the improvements that you would like to see?
We have not yet done any project under the Sakigake framework, but we are looking to be involved in the system. It is also difficult to point out possible improvements that can be done in the medical system since different countries have different regulations. We need to work within Japan's regulatory framework, and our concern is to work for cancer patients within the given framework in each country.
Japan is the oldest society in the world and it has a rapidly shrinking population which presents challenges such as a labor crisis. Conversely, the aging population also creates an interesting scenario for pharmaceutical and medical companies. What challenges and opportunities has Japan's demographic change presented to OncoTherapy?
OncoTherapy specializes in cancer treatment. Since the risk of people getting cancer above 50 years old increases drastically, we believe there is a growing market for our business. Securing talented workers and personnel is crucial for our company's sustainability. With COVID-19 for the past three years, remote working has become the new work style and this allows us to keep hiring older people. At the same time, we are actively recruiting foreign employees for our subsidiaries.
Although there are various negative aspects to having an aging population, our business is not impacted much by it. It actually presents positive opportunities for us. The choice of cancer treatments varies depending on age. The younger generation can withstand surgeries and radiation treatments that are quite harsh on the body whereas the same cannot be said for older patients. There would be more need for effective and safe treatment that focuses on the elderly. We have to be flexible in adapting and introducing new cancer treatments and management based on age.
One of the big limits in oncology and cancer treatments is that the mainstream practice is chemotherapy, which destroys both cancer and normal cells, resulting in plenty of adverse effects on patients. Looking to the future, what avenues for cancer treatment do you see? What new methods and better technologies do you think will become more prevalent in the future?
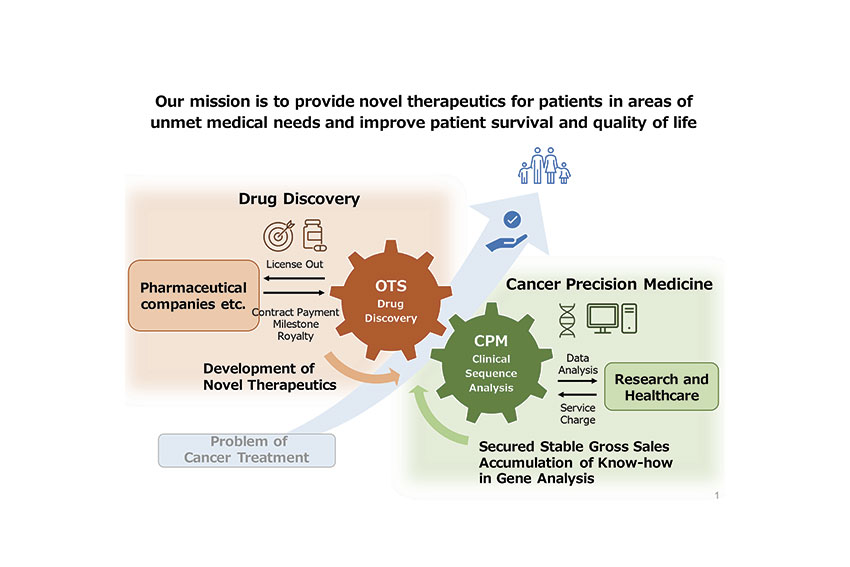
In general, surgery, chemotherapy, and radiotherapy are regarded as 3 major cancer treatments. Immunotherapy is considered the fourth oncology therapy and there is a huge potential in Japan, however, this has been prioritized in the US and the European market. Our subsidiary called CPM (Cancer Precision Medicine, Inc.) does various genetic and immunologic analyses on the creation of vaccination for immunotherapy. There are fewer side effects and adverse reactions with this medication. If we promote the advancement of immunotherapy, this could be the best option for cancer treatment. The challenge in cancer therapy is that all cells including normal cells are attacked so many people are suffering from adverse reactions. We are thinking about two important keywords. The first one is molecular target therapy which targets only cancer cells. The other one is personalized medicine. Our subsidiary is conducting genome analysis to profile genetic information for individual patients. We think that personalized therapy would be another effective cancer therapy in the near future.
We know that your firm was founded in 2001 as a drug discovery specialist that focuses on the cancer-specific molecular target to treat cancers. Can you give us an introduction to molecular-targeted therapy agents and how they help in treating cancer?
We identified various novel molecular targets and have developed molecular target agents that only attack cancer cells and do not adversely affect normal cells. Conventional treatments often have severe side effects like hair loss, nausea, and blood disorders. Molecular target therapy has fewer negative impacts on the body. We have unique technologies that can extract only cancer cells at high purity and thus can analyze them and develop cancer-specific drugs. There are two important considerations, the first is the technology and the second is our selection criteria. In terms of technology, we isolate only cancer cells from tumor tissue. If cancer cells are present in a big tumor, other research scientists or companies usually analyze the tumor after the breakdown of all different kinds of cells. However, we isolated only cancer cells through pathological methods. This allows us to examine pure cancer cells and highly accurate cancer gene expression patterns. The second one is the selection criteria for novel molecular targets. Our main three criteria include; First, our new target should be highly expressed in many types of cancer. Second, genes should not be expressed in normal organs because we want to clear out adverse reactions. The third is a functional analysis to validate whether the molecular target plays an essential role in cancer cell survival or growth.
Developing innovative technology and medicine in oncology have long lead times, but they are also often titled miracle innovations. In the medical field, clinical trials oftentimes fail even though they are promising during preclinical trials. Why do you believe this is, and why do these lab-based medicines fail when it comes to human-based trials?
The simple reason is that animal study and human study are completely different, and human physiology is more complex. Furthermore, genes differ from one person to another, so a drug that is effective for person A might be ineffective for person B. It is important to develop a balanced drug for wide use or personalized medicine. Our subsidiary CPM is supporting the drug discovery, drug choice and development of personalized medicine. We want to keep pursuing personalized medicine to help cancer patients as much as possible.
Can you tell us a bit more about personalized medicine? How are you planning to achieve such a level of personalization, and how are you able to understand what each patient needs?
In oncology treatment, the total genome of the person can be first analyzed through an algorithm that determines the specific gene alterations and provides helpful information to choose treatment direction. If a sudden emergence of cancer cells occurs, we can search for existing and conventional drugs which mostly fit for the patient’s genetic background. If it does not exist, a personalized peptide will be developed for treating the tumor with specific mutations. Our subsidiary CPM is doing two approaches in the genetic analysis of cancer.
First, if a patient has a specific gene mutation, for example, that may increase specific enzyme activity in cancer cells, some pharmaceutical companies might have developed drugs that could inhibit such function, and we can match the mutation information with the selection of cancer drugs.
The second approach is doing a whole genome or whole exome analysis to look for all kinds of mutations in cancer cells. If the patient, unfortunately, does not have any type of specific mutation matched with a drug, we can think of designing specific cancer antigens. Because some genetic mutations can be recognized by our immune cells as an antigen, we can provide peptides, a group of amino acids, according to the mutation profile. Then, our immune cells can recognize the same antigens on cancer cells, and fight with them. Therefore, according to the cancer mutations in each patient, our two approaches help look for mutation-matched cancer drugs or provide new cancer antigen immunotherapies.
One of the antibody drugs you are developing is the cancer therapy antibody OTSA101 for treating synovial sarcoma. It is currently undergoing phase one clinical trials in Japan. What are your expectations for this clinical trial?
OTSA101 is currently under phase one trial in Japan, and because of legal regulation, we cannot disclose details about the clinical trial. An important keyword is “orphan drug” as synovial sarcoma is a rare disease. It occurs in 1–3 people out of 1,000,000 per year. Big pharmaceutical companies usually have little interest in rare diseases because it is a small market. However, we think that our new technologies from cancer genome analysis and molecular target therapies might be important to save cancer patients from rare diseases. Of course, we also need to think about the business and the sales aspect. For example, we hope to make the approval applications passable because this is an orphan drug necessary for rare diseases. In addition, we can expand and produce antibodies for other types of cancer because OTSA101’s molecular target is highly expressed in many other types of cancer.
Are you looking to have similar partnerships in overseas markets?
Yes, we are. We have been focusing mainly on oncology and looking for overseas partners in this field. Moreover, we have conducted basic research that could be applied to other diseases based on our accumulated experience and compound library. We want to fully utilize this library to reanalyze our data and make it valuable for our potential partners. Our information and technologies can be expanded globally, so we are looking into having global partnerships.
In 2010, you opened a France subsidiary that is now closed. In 2017, you created a joint venture called Cancer Precision Medicine with South Korean firm Theragen Bio. Moving forward, are there any countries or regions you want to expand into? What strategies will you employ to do so?
Our global strategy is to work together with the OTS Group. With OncoTherapy, we focus on new overseas partnerships. With CPM, we want to pursue the development of a test kit to be sold in the domestic and global markets. With our existing compound library and database, we want to find the best way to partner with overseas pharmaceutical companies and sell the data as a business model. There are two approaches from OncoTherapy and CPM. First is the development of novel molecular targeted therapies for cancer. Second, is the genetic analysis and development of test kits. With these, we can create a synergy effect and ultimately can help the patients to treat cancer as well as other types of disease. We think about maximizing synergy within the OTS Group and expanding globally.
Imagine that we come back on the last day of your presidency to interview you again. What objective or ambition would you like to have achieved during your time as President?
Since I am still in the starting phase, it is hard to imagine the goals and outcomes at this moment. Our company's mission is to provide a new type of effective treatment for cancer patients with fewer side effects, as soon as possible. When you return on my last day, I will be able to let you know about all the progress we have made. Although we already have some accomplishments, we want to keep improving.
0 COMMENTS